CEQ – Corrosion Studies Kit
Corrosion represents a significant factor in determining durability and safety of industrial processes. Student engineers need to fully understand the effects of corrosion and how these can be anticipated and prevented.
Description
Corrosion represents a significant factor in determining durability and safety of industrial processes. Student engineers need to fully understand the effects of corrosion and how these can be anticipated and prevented.
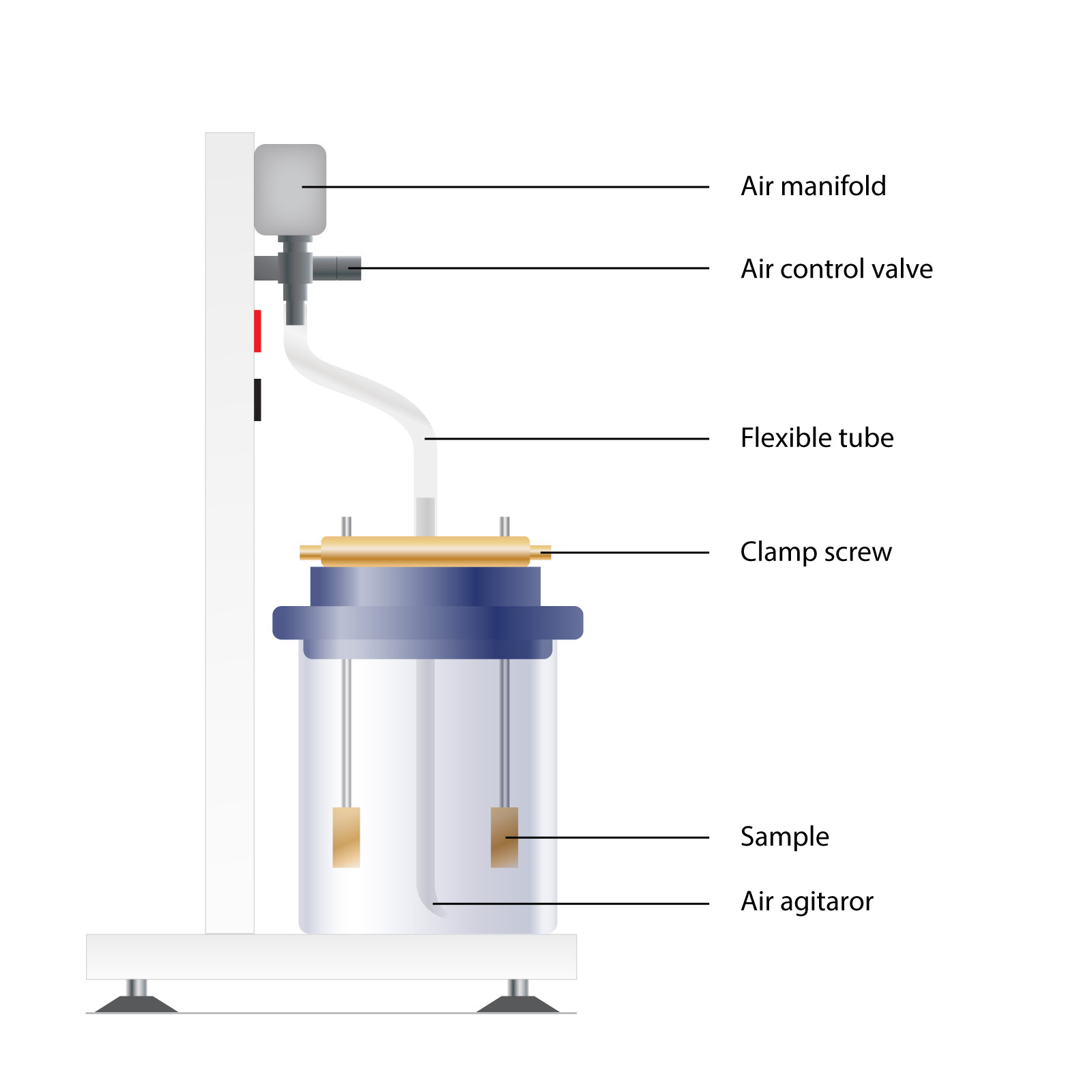
The Armfield corrosion studies kit uses a number of simple items of equipment in a series of tests, designed to demonstrate to the student how potentially corrosive situations may be recognised and avoided. Although the experiments refer principally to steel water systems, the apparatus may be used as a test bench for other chemical systems.
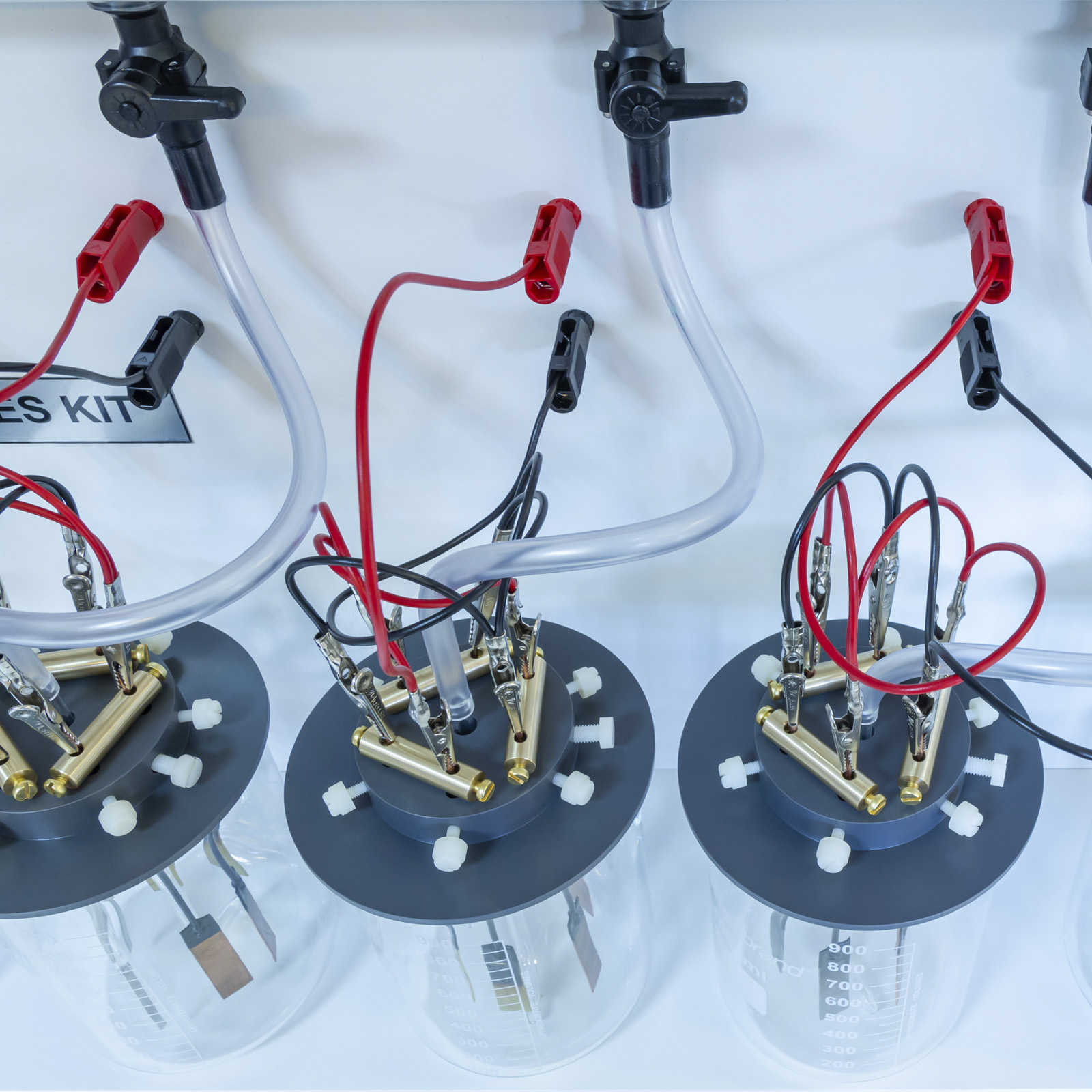
The equipment allows for the simultaneous study of up to eight corrosion cells of whatever type is selected according to the teaching syllabus being followed. Each test cell allows for the immersion of three similar test specimens in the test liquid at any one time, to eliminate ‘rogue’ results from atypical metal samples. Each sample is mounted in a manner that minimises secondary effects and the metal surface of known area is exposed to the test liquid.
Corrosion rates are measured both by visual observation and direct weighing after a known period of immersion. Stirring is by air or inert gas agitation.
All connecting glass and plastic tubing is provided, as are the appropriate supports for the specimens and glass test cells. A digital pH meter and microsensor is supplied to ensure the correct strength of initial test solutions. For the study of electrochemical corrosion effects, a low-voltage supply is included together with all necessary electrical connections. Initial buffer powders of pH 4, 7 and 9 are supplied.
The apparatus requires approximately 2m2 of laboratory bench space for experimentation by two students. A full instruction manual is provided explaining how each corrosion cell situation is set up and the results assessed.
Technical Specifications
Power supply unit:
Outputs:
0-15V at 2A
0-30V at 1A
Air pump: Diaphragm type
Air flow rate: 6 l/min
Max head: 0.8 kg/cm²
Max power of motor: 0.05kW
Digital pH microsensor and pH meter:
Range of pH meter: 0-14pH
Resolution: 0.01pH
Accuracy: ±0.01pH
Dimensions: 195 x 29 x 15mm
Beaker (x8): Borosilicate glass (Pyrex)
Capacity: 1,000ml
Electrodes: Platinum
Test piece samples: Zinc, mild steel, copper, brass
Features & Benefits
Downloads
- The influence of pH on corrosion – To examine the effect of pH on the rate of corrosion of steel.
- Effect of dissolved oxygen concentration – To examine the effect of brine (sodium chloride solution) with or without the presence of oxygen, on the corrosion of steel.
- Galvanic action – To demonstrate the effect of electrically connecting together two dissimilar metals in a solution to form an electro-chemical cell.
- Electrolytic corrosion – To show that stray voltages between metals in a corrosive environment can greatly affect the rates of corrosion.
- Cathodic protection by impressed voltage – To demonstrate that the setting up of a ‘corrosion cell’ with dissimilar metals in contact may be opposed by applying a voltage from an external power supply.
- Corrosion Inhibition – To show that the corrosion of steel may be reduced by the use of certain chemicals (inhibitors).
- Stress Corrosion – To examine the effect of internal stress on the corrosion rate of steel.
- Prevention of scaling
The influence of pH on corrosion
Iron and steel corrode in acid environments but much less so in alkaline situations. Students carry out corrosion tests at values of pH 4, 7 and 9. The results are explained with reference to the electrode potential series.
Stress corrosion
Metal samples are stressed in a variety of simple ways, including bending, scratching, filing or drilling. The alteration of the metal crystal structure may explain the observed results.
Brine and oxygen environments
Oxygen may be artificially removed from water by nitrogen purging. Comparison of corrosion rates of metal samples in oxygenated and deoxygenated water may be related to localised ionic corrosion cells. The effects of sodium chloride solutions on the cells may also be deduced.
Galvanic action
Dissimilar metal pairs of steel/copper, and steel/zinc, are connected to form an electric circuit. The subsequent corrosion may be explained by reference to the electrode potential series.
Cathodic protection by impressed voltage
If platinum and steel are electrically connected, steel becomes the anode and corrodes in water. An external voltage may be applied to reverse the effect, thus protecting the steel.
Electrolytic corrosion
This demonstration shows that stray voltages between connected, dissimilar metals can greatly affect the corrosion rates. The low-voltage DC supply is connected to various combinations of steel samples with platinum, zinc or copper samples.
Corrosion inhibition
The passivating influence of certain phosphates and silicates in solution on corrosion rates of steel may be demonstrated.
Water treatment projects
The following demonstrations may be conducted with the apparatus:
- Calcium carbonate stabilisation
- Oxidation of iron and manganese in ground waters
- Disinfection of waste water with chlorine solutions
- Water softening by chemical precipitation
- The corrosion studies kit enables simultaneous study of up to eight corrosion cells each containing three test specimens
- Sample mounting in a manner to minimise secondary effects
- Corrosion rates measured visually and by weighing
- Stirring by air or inert gas
- A service panel
- Air pump
- Eight test cells of glass construction, with specially machined lids enabling samples to be mounted
- Test cell supports
- Digital pH meter
- Specimen pieces of steel, zinc, brass and copper
- Platinum electrodes
- Low-voltage supply
- Associated glassware, tubing and wiring
To be supplied by user:
- Electronic top-loading balance
- Cartridge de-ioniser
- Oven
- Laboratory glassware
- Inert gas cylinder eg. nitrogen – Smallest available size (pressure regulator needed)
Electrical supply:
CEQ-A: 220-240V / 1ph / 50Hz / 12A
CEQ-B: 120V / 1ph / 60Hz / 14A
CEQ-G: 220-240V / 1ph / 60Hz / 12A
Consumables
17g Potassium iodine
10 ml Hydrochloric acid
60g Sodium chloride
8 litres De-Ionised water
PACKED AND CRATED SHIPPING SPECIFICATIONS
Volume: 0.5m³
Gross Weight: 80Kg
Length: 1.19m
Width: 0.28m
Height: 0.43m
- CEQ-A
- CEQ-B
- CEQ-G